In China, deregulation has led to a rapid increase in clinical trials of pharmaceuticals, and the number of new drug developments has grown to a level comparable to that of the United States.
China's development in science and technology is remarkable, and it has become one of the world's leading influences in several fields, including AI. China is also gaining influence in the field of pharmaceutical development, and the science media
China's Clinical Trial Boom - by Hiya Jain - Asimov Press
https://www.asimov.press/p/china-trials
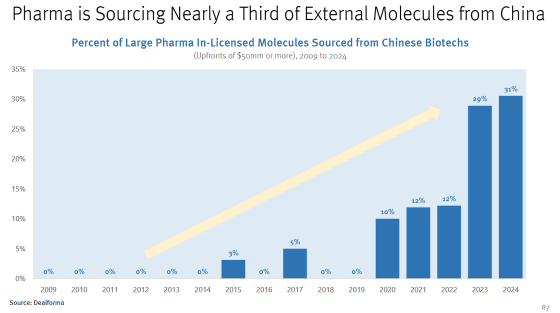
Major pharmaceutical companies often develop and manufacture drugs not only by purchasing molecules developed in-house, but also by purchasing molecules developed by other companies and research institutes. The graph below shows the trend in the proportion of molecules developed in China among the molecules purchased by major pharmaceutical companies. The proportion of purchases from China has increased sharply since around 2020, and in 2024, 31% of the total were molecules purchased from China.
Below is a graph comparing the number of clinical trials started in China (red) and the United States (blue). The number of clinical trials started in China was about 600 per year in 2017, but by 2023, it had more than tripled to about 2,000.
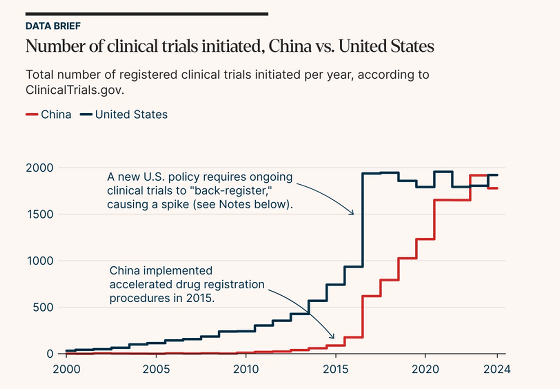
The increase in clinical trials in China is thought to be due to deregulation. In 2017, China's drug regulatory authority, NMPA, introduced a regulation stating that 'if the regulatory authority does not raise an objection within 60 days, a clinical trial is deemed approved.' Furthermore, China joined
Another characteristic of clinical trials in China is the large number of subjects. The graph below shows the number of subjects in clinical trials divided into '1-100 (white),' '101-500 (red),' '501-1000 (yellow),' and 'more than 1000 (blue).' In the United States, three-quarters of clinical trials had between 1 and 100 subjects, while in China, more than 40% of clinical trials had more than 101 subjects.
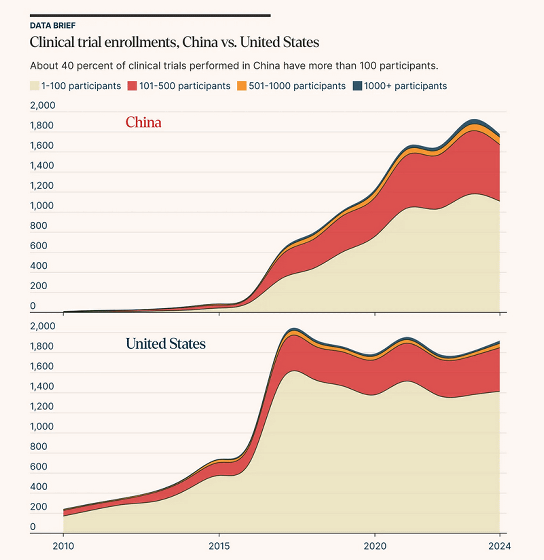
Asimov Press points out that 'the United States is a pioneer in modern drug development, but is currently showing signs of stagnation. Meanwhile, China has reformed its clinical trial process to maximize efficiency and achieve rapid progress,' arguing that measures such as shortening the time needed to start clinical trials and making better use of foreign data are necessary.
Related Posts:
in Science, Posted by log1o_hf